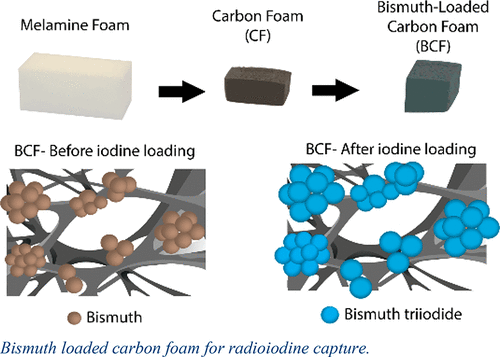
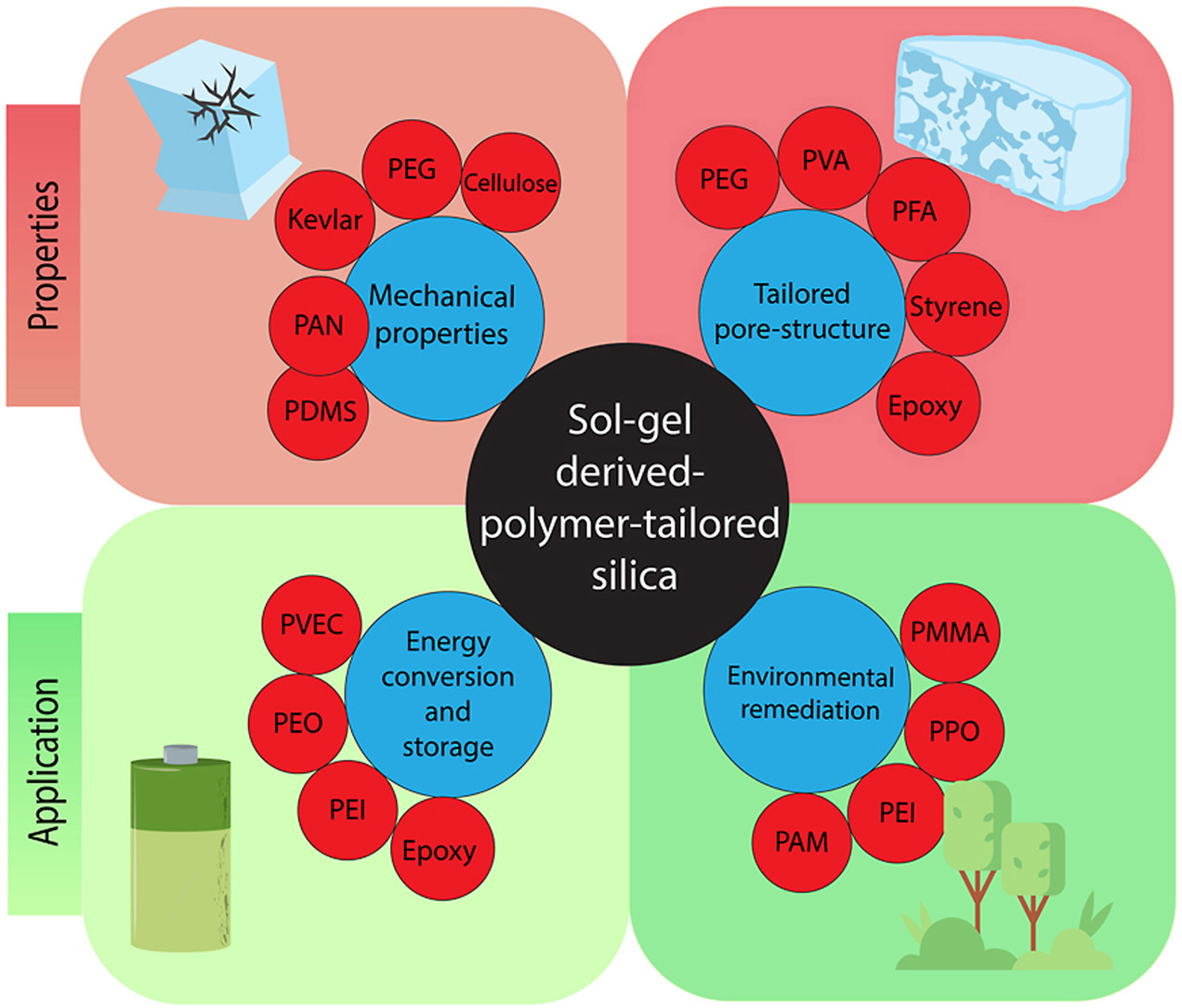
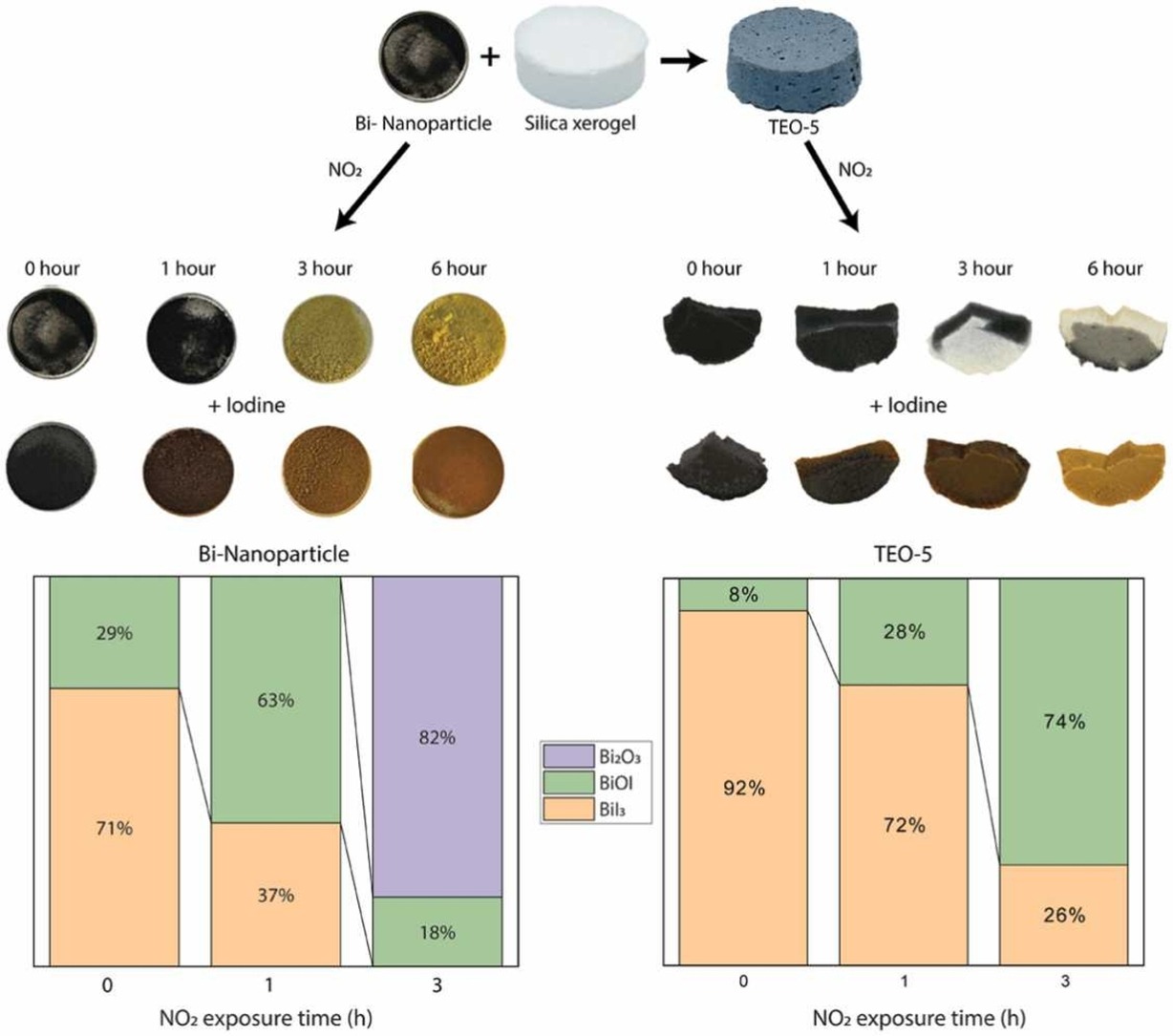
About Me
I am a Research Scientist in the Department of Chemical and Materials Engineering at the University of Nevada, Reno, where I also manage the Electron Microscopy and Microanalysis Facility (EMMF). My research focuses on nanoscience and nanotechnology, particularly in the development of high specific surface area materials for gas adsorption and rheological applications. I specialize in nanoscale synthesis, wet chemistry, and advanced material characterization techniques including electron microscopy, surface analysis, and adsorption measurements to study material behavior at the atomic and molecular scale.
Previously, I served as an Assistant Teaching Professor at UNR for two years, teaching core undergraduate and graduate courses in materials science and mentoring students in both classroom and research settings. I remain committed to advancing high impact research while promoting inclusive, hands on learning experiences. My work supports interdisciplinary collaboration across academia and industry with ongoing contributions to peer reviewed publications and research development.
In my previous research, I worked with vacuum systems and thin film deposition to study surface effects such as the magneto-optic Kerr effect and spin-polarized electron behavior. This work focused on understanding magnetic and electronic properties at the nanoscale, using surface-sensitive techniques to explore thin film interfaces and their role in spintronic applications.
Contact Details
Karthikeyan Baskaran
Reno, NV USA
karthikeyan (@) baskaran.me